Compound (1) was identified by scientists at Merck as a potent Aurora kinase inhibitor with the potential to be useful in the treatment of various cancers. In this paper the authors reveal that the basic amine which is key to good cellular activity in this series results in rapid metabolism (mainly via N-dealkylation) and poor oral absorption. Attempts to obtain better oral pharmacokinetic profiles by preparing various prodrugs were unsuccessful but approaches which looked to block metabolism around the amine group as well as introduce substituents that reduced the basicity of the amine led to significant improvements in pharmacokinetics without significantly compromising cellular activity.
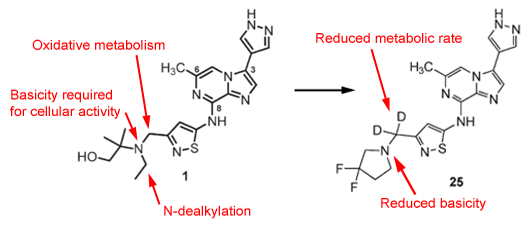 The 3,3-difluoropyrrolidine analogues (e.g. see (25)) were one of the better modifications in this position giving a good balance of enhanced exposure and cellular potency. However, whilst some of these types of compound demonstrated improved PK in mouse, rat, and dog, all had poor PK profiles in monkey. Metabolic profiling in monkey hepatocytes using tritium-labelled inhibitor demonstrated that benzylic oxidation was an issue in this species. In order to attempt to block this and enhance exposure the effects of benzylic deuteration were explored. Relying on the kinetic isotope effect this has been previously reported as a way of slowing down metabolism at vulnerable sites but there are still relatively few real examples in the literature. In this instance not all deuterated analogues had improved exposure in rats but in those cases where an improvement was noted, a 2- to 5-fold reduction in the rate of clearance was observed. In the case of compound (25) this improvement also translated to monkey where a 3-fold increase in oral exposure was observed relative to the non-deuterated parent. Compound (25) subsequently demonstrated robust activity on oral dosing in a xenograft model. It will be interesting to see if this type of approach ultimately leads to clinical candidates.
The 3,3-difluoropyrrolidine analogues (e.g. see (25)) were one of the better modifications in this position giving a good balance of enhanced exposure and cellular potency. However, whilst some of these types of compound demonstrated improved PK in mouse, rat, and dog, all had poor PK profiles in monkey. Metabolic profiling in monkey hepatocytes using tritium-labelled inhibitor demonstrated that benzylic oxidation was an issue in this species. In order to attempt to block this and enhance exposure the effects of benzylic deuteration were explored. Relying on the kinetic isotope effect this has been previously reported as a way of slowing down metabolism at vulnerable sites but there are still relatively few real examples in the literature. In this instance not all deuterated analogues had improved exposure in rats but in those cases where an improvement was noted, a 2- to 5-fold reduction in the rate of clearance was observed. In the case of compound (25) this improvement also translated to monkey where a 3-fold increase in oral exposure was observed relative to the non-deuterated parent. Compound (25) subsequently demonstrated robust activity on oral dosing in a xenograft model. It will be interesting to see if this type of approach ultimately leads to clinical candidates.

